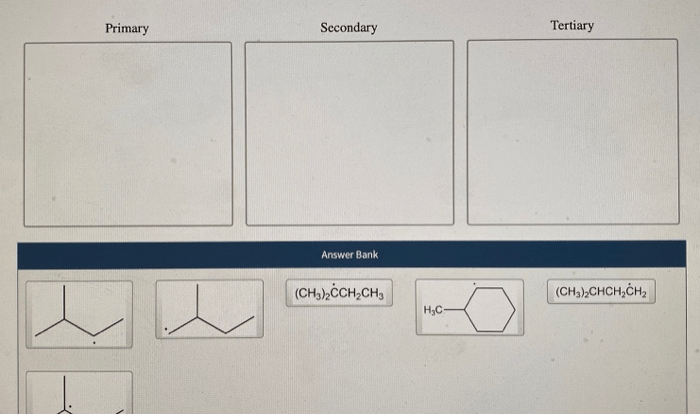
Rank the isotopes from most to fewest neutrons – Embarking on a journey into the realm of isotopes, we unravel the intriguing relationship between elements and their neutron content. By examining the neutron number’s profound impact on isotope stability and properties, we embark on a quest to rank isotopes from the neutron-richest to the neutron-poorest.
Delving deeper into the intricacies of isotope notation and the concept of mass and atomic numbers, we lay the groundwork for a comprehensive understanding of isotope classification.
Isotope Overview
Isotopes are variants of an element that have the same atomic number but different neutron numbers. The atomic number determines the element’s identity and the number of protons in the nucleus. Isotopes are represented by their mass number, which is the sum of the number of protons and neutrons in the nucleus.
Neutron Number

The neutron number of an isotope is the number of neutrons in its nucleus. It affects the isotope’s stability and properties. Isotopes with a large neutron-to-proton ratio tend to be less stable and undergo radioactive decay.
Ranking Isotopes
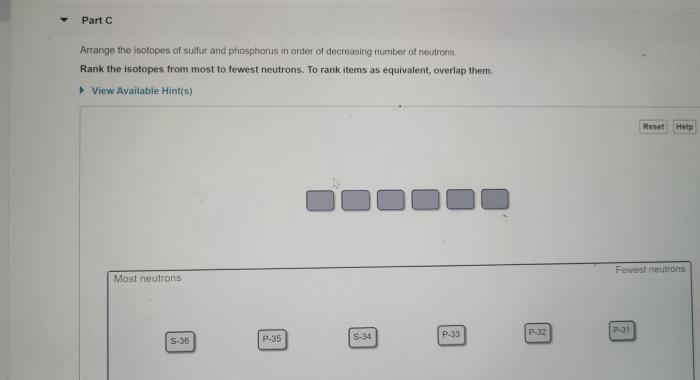
| Isotope Symbol | Element Name | Mass Number | Neutron Number | Stability |
|---|---|---|---|---|
| 1H | Hydrogen | 1 | 0 | Stable |
| 2H | Hydrogen | 2 | 1 | Stable |
| 3H | Hydrogen | 3 | 2 | Radioactive |
| 12C | Carbon | 12 | 6 | Stable |
| 14C | Carbon | 14 | 8 | Radioactive |
Isotopes are ranked from most to fewest neutrons by their mass number. Isotopes with higher mass numbers have more neutrons.
Applications
Isotopes have various practical applications based on their neutron numbers. For example:
- 14C is used in radiocarbon dating to determine the age of organic materials.
- 235U is used as fuel in nuclear reactors.
- 99mTc is used in medical imaging procedures.
User Queries: Rank The Isotopes From Most To Fewest Neutrons
What factors influence the stability of isotopes?
The neutron-to-proton ratio plays a crucial role in determining isotope stability. Isotopes with a balanced neutron-to-proton ratio are generally more stable.
How are isotopes used in practical applications?
Isotopes find widespread use in medicine (e.g., radioactive isotopes for cancer treatment), industry (e.g., isotope tracing in manufacturing processes), and research (e.g., carbon-14 dating in archaeology).