Unveiling the solubility rules worksheet with answers, this comprehensive guide embarks on a journey to unravel the intricacies of solubility, equipping learners with the knowledge to predict the solubility of compounds effortlessly.
Delving into the fundamental principles of solubility rules, we elucidate their significance in chemistry and showcase their practical applications across diverse fields, from industry to medicine and environmental science.
Solubility Rules: Solubility Rules Worksheet With Answers
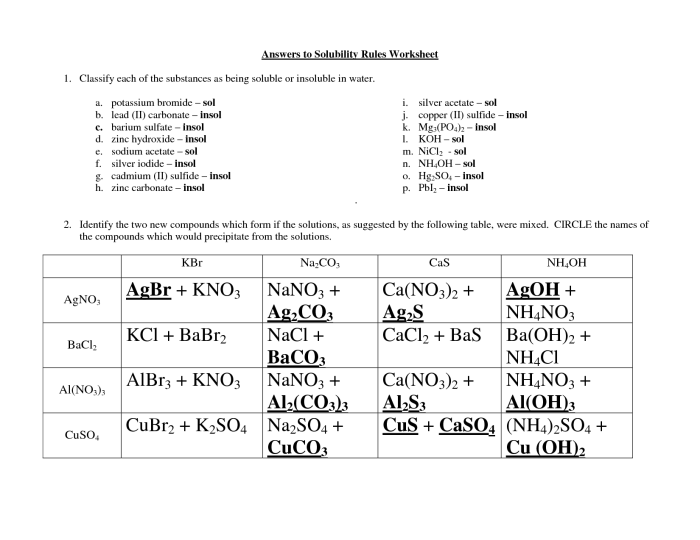
Solubility rules are a set of guidelines that help predict whether a compound will dissolve in a particular solvent. They are based on the chemical properties of the compound and the solvent.
Solubility rules are important in chemistry because they allow us to predict the behavior of compounds in solution. This information can be used to design experiments, develop new materials, and understand the interactions between different substances.
Here are some real-life examples of solubility rules:
- Salts are generally soluble in water.
- Acids are generally soluble in water.
- Bases are generally soluble in water.
- Organic compounds are generally insoluble in water.
- Nonpolar compounds are generally insoluble in water.
Types of Solubility Rules
There are two main types of solubility rules:
- Ionic solubility rulespredict the solubility of ionic compounds in water.
- Covalent solubility rulespredict the solubility of covalent compounds in water.
Ionic solubility rules are based on the charges of the ions in the compound. Covalent solubility rules are based on the polarity of the compound.
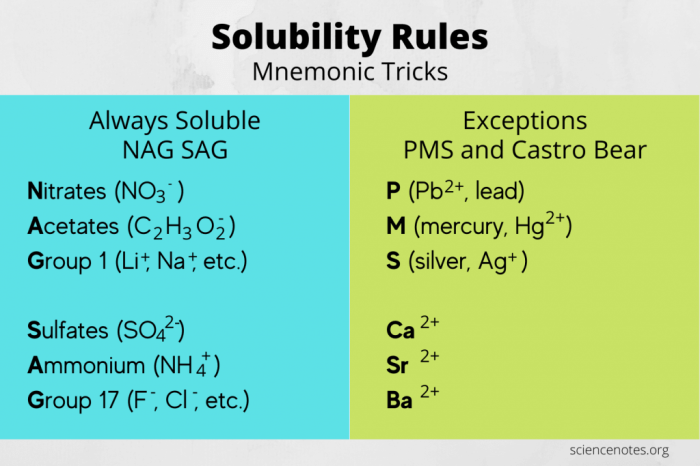
Here are some examples of ionic solubility rules:
- All Group 1 cations (Li+, Na+, K+, Rb+, Cs+) are soluble.
- All Group 2 cations (Ca2+, Sr2+, Ba2+) are soluble, except for BaSO4.
- All ammonium (NH4+) cations are soluble.
- All nitrate (NO3-) anions are soluble.
- All chloride (Cl-) anions are soluble, except for AgCl, PbCl2, and Hg2Cl2.
Here are some examples of covalent solubility rules:
- Polar covalent compounds are generally soluble in water.
- Nonpolar covalent compounds are generally insoluble in water.
- Compounds with hydrogen bonding are generally soluble in water.
Solubility Rules Worksheet
Here is a solubility rules worksheet with answers:
- Predict the solubility of NaCl in water.
- Predict the solubility of CaSO4 in water.
- Predict the solubility of NH4Cl in water.
- Predict the solubility of CH3OH in water.
- Predict the solubility of C6H6 in water.
- NaCl is soluble in water.
- CaSO4 is insoluble in water.
- NH4Cl is soluble in water.
- CH3OH is soluble in water.
- C6H6 is insoluble in water.
Applications of Solubility Rules, Solubility rules worksheet with answers
Solubility rules have a wide range of applications in various fields, including:
- Industry: Solubility rules are used to design and optimize chemical processes, such as crystallization, precipitation, and extraction.
- Medicine: Solubility rules are used to develop new drugs and understand the interactions between drugs and the body.
- Environmental science: Solubility rules are used to assess the environmental impact of chemicals and to develop remediation strategies.
- Everyday life: Solubility rules are used to understand the behavior of everyday substances, such as salt, sugar, and soap.
Detailed FAQs
What is the purpose of a solubility rules worksheet?
A solubility rules worksheet provides a structured framework for students to practice applying solubility rules to predict the solubility of different compounds.
How can solubility rules be applied in everyday life?
Solubility rules find applications in various aspects of daily life, such as understanding the behavior of medications, predicting the formation of precipitates in water systems, and optimizing cleaning processes.